Coronary Interventions
Coronary interventions aim to restore blood flow in blocked or narrowed coronary arteries to improve heart
function and relieve symptoms of coronary artery disease
Access Sites: Transradial and Transfemoral
The choice of vascular access impacts patient comfort, complication rates, and procedural success.
Transradial Access
- Access via the radial artery at the wrist
- Advantages:
- Lower bleeding and vascular complication rates.
- Enhanced patient comfort and early ambulation.
- Considerations:
- Smaller artery may limit catheter size (typically 6F or smaller).
- Possible anatomical variations requiring specialized catheters.
- Potential tortuosity of proximal upper extremity vessels that may challenge the steerability and torqueability of the catheter while attempting to engage the coronary
- Potential for conversion to groin access site if limited by spasm or need for more catheter support
Transfemoral Access
- Access via the femoral artery in the groin
- Advantages:
- Accommodates larger sheath sizes.
- Essential for structural heart interventions and complex PCIs requiring larger devices.
- Considerations:
- Higher risk of bleeding and vascular complications.
- Requires longer bed rest post-procedure.
Catheters for Coronary Engagement
Proper catheter selection is essential for successful coronary interventions.
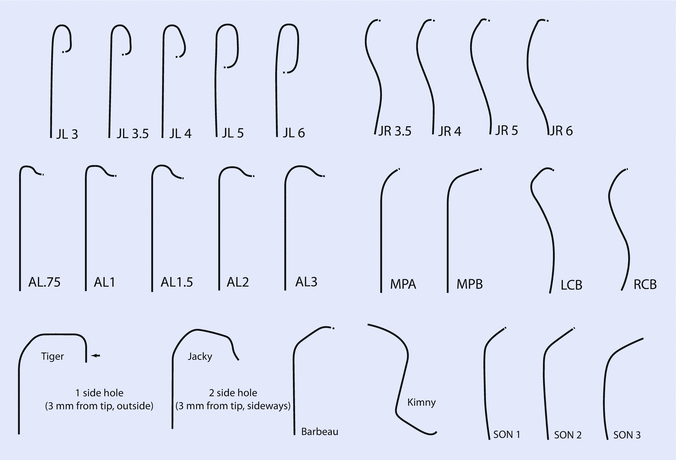
- Judkins Catheters:
- Standard curves (JL4, JR4) for most anatomies.
- Available in sizes from 5F to 8F.
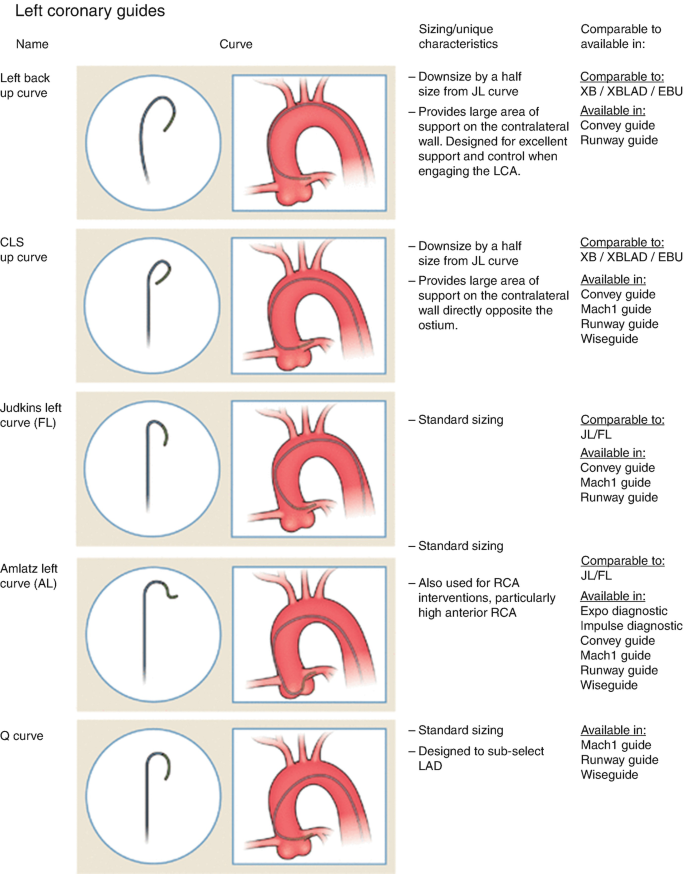
- Extra Backup (XB/EBU) Catheters:
- Provide additional support for complex or tortuous lesions.
- Commonly used for left coronary interventions.
- Amplatz Catheters:
- Useful for anomalous origins or when additional support is needed.
- Radial-Specific Catheters:
- Examples include Tiger and Jacky catheters.
- Designed for both left and right coronary engagement via radial access.
Guide Extension Catheters
Devices like the GuideLiner™ (Teleflex) or TrapLiner™ assist in device delivery:
- Provide deep intubation into the coronary artery.
- Facilitate delivery of balloons and stents in challenging lesions.
- Compatible with standard guiding catheters.
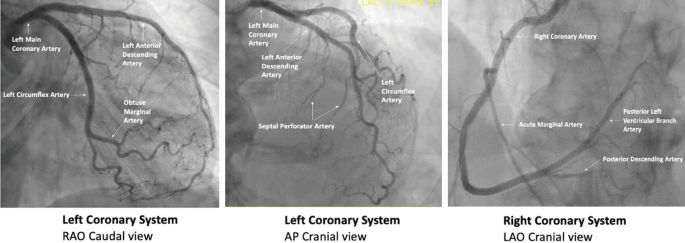
Angiographic Views and Projections
Accurate visualization of coronary anatomy is essential for diagnosis and intervention. Here are some tips to help guide you:
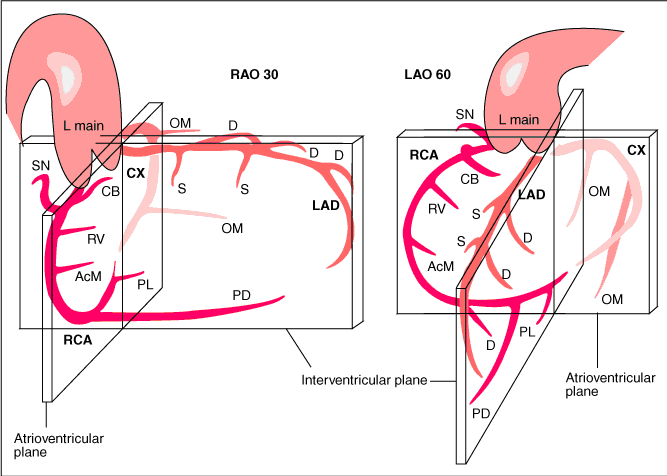
- RAO views make the catheter appear right of the spine while LAO views make the catheter appear left of the spine
- Cranial views help you visualize distal vessel especially for the LAD on the left coronary diagnostic
- Caudal views help you visualize proximal segments of a vessel
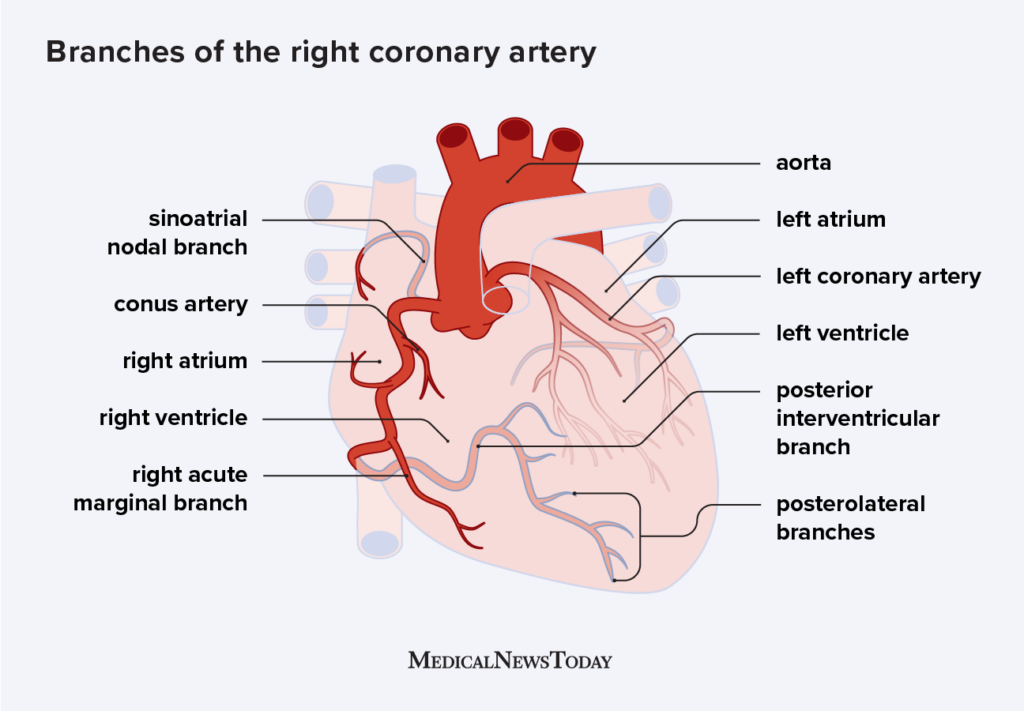
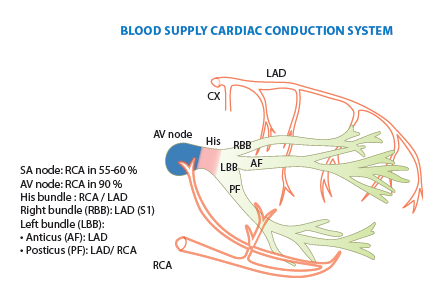
- When engaging the RCA, adding cranial to LAO view helps separate the distal bifurcation of the PDA and RPL
- In RAO caudal, the circumflex is coming down and towards you. In AP cranial or LAO cranial, the LAD is the center of focus - it comes down and tapers to the apex
- For RCA, the RV marginal branch are the branches that precede the bifurcation. After the distal RCA bifurcation, then the branches are posterolateral or PDA
- Proximal LAD ends at the take off of the first diagonal branch then it transitions into mid LAD
- Ways to distinguish LAD: identify septal perforator branches, and remember, the LAD is not border forming
Balloons for Coronary Interventions
Balloons are critical tools in percutaneous coronary intervention (PCI), used for lesion preparation and stent deployment.
Types of Balloons
- Compliant Balloons:
- Made from flexible materials like nylon or polyurethane.
- Expand more with increased pressure, allowing for vessel sizing.
- Used primarily for initial lesion dilatation.
- Non-Compliant Balloons:
- Constructed from rigid materials such as polyethylene terephthalate (PET).
- Resist expansion beyond their nominal diameter even at high pressures.
- Ideal for high-pressure post-dilation to optimize stent expansion.
- Scoring and Cutting Balloons:
- Feature specialized surfaces (micro-blades or scoring elements).
- Modify plaque by creating controlled dissections.
- Useful for resistant or fibrotic lesions.
Manufacturers and Specifications
Leading balloon manufacturers include:
- Boston Scientific: Offers the Emerge™ PTCA Dilatation Catheter series.
- Available in diameters from 1.2mm to 5.0mm.
- Lengths range from 6mm to 30mm.
- High-rated burst pressures (RBP) up to 20 atm for non-compliant models.
- Abbott Laboratories: Provides the Trek™ and NC Trek™ Balloon Catheters.
- Diameters from 1.2mm to 5.0mm.
- Lengths from 6mm to 30mm.
- NC Trek™ non-compliant balloons with RBPs up to 24 atm.
- Medtronic: Offers the Sprinter Legend™ and NC Euphora™ Balloon Catheters.
- Wide range of sizes and lengths.
- Advanced trackability and pushability features.
Bare Metal Stents (BMS) and Drug-Eluting Stents (DES)
Stents scaffold the artery to prevent recoil and restenosis after angioplasty.
Types of Stents
- Bare Metal Stents (BMS):
- Constructed from stainless steel or cobalt-chromium alloys.
- Provide mechanical support without drug coating.
- Higher risk of restenosis compared to DES.
- Drug-Eluting Stents (DES):
- Coated with antiproliferative drugs like everolimus or zotarolimus.
- Reduce neointimal hyperplasia and restenosis rates.
- Current-generation DES have thin struts and biocompatible polymers.
Manufacturers and Stent Specifications
Major stent manufacturers and their offerings include:
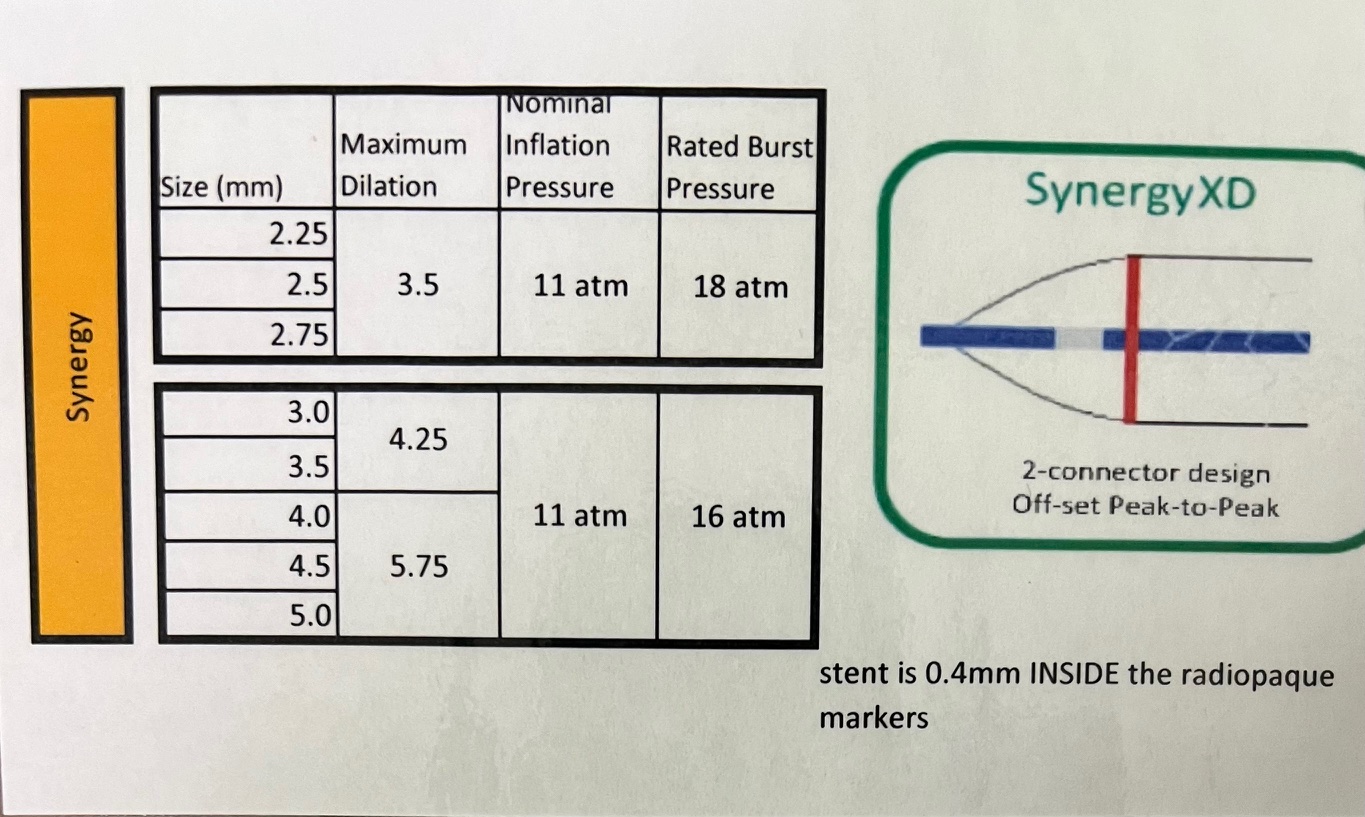
- Boston Scientific:
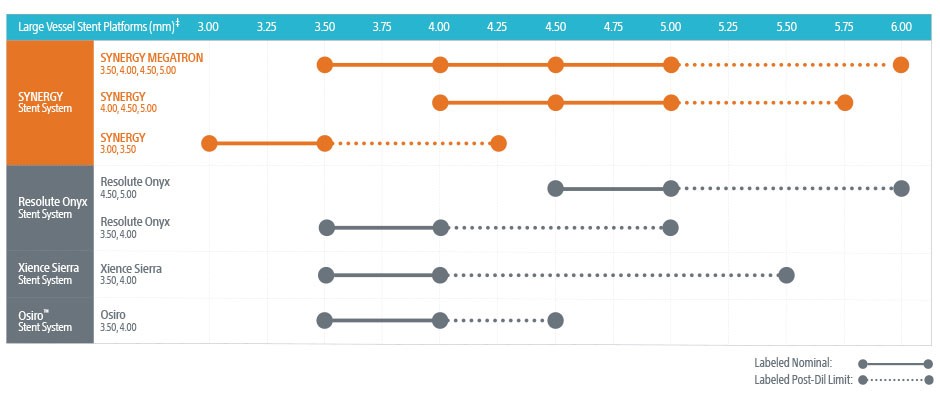
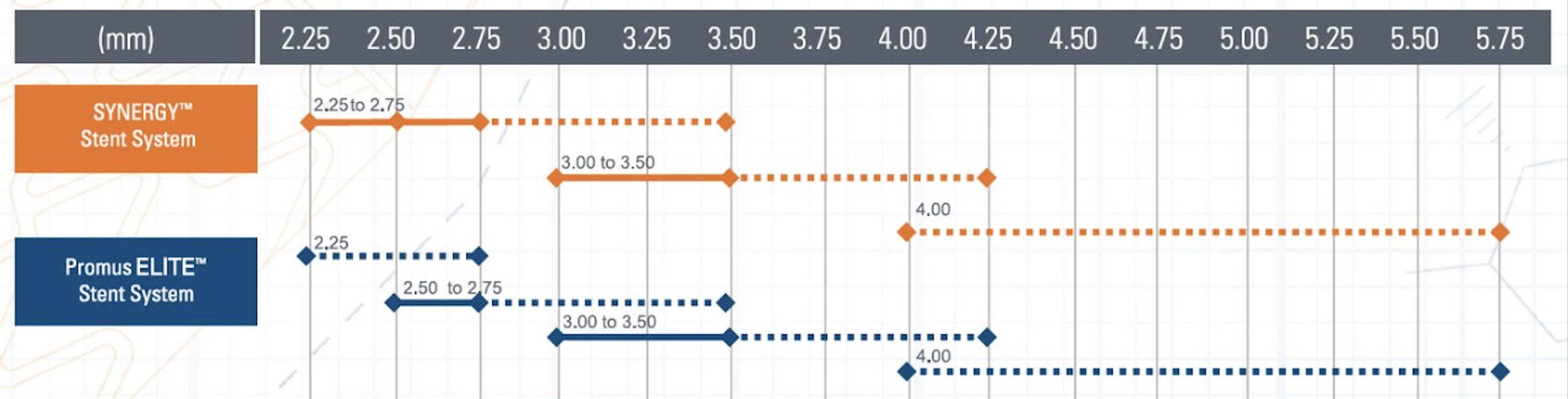
- SYNERGY™ Everolimus-Eluting Platinum Chromium Stent:
- Diameters: 2.25mm to 5.0mm.
- Lengths: 8mm to 38mm.
- Ultra-thin 74µm struts.
- Bioabsorbable polymer coating.
- Nominal Pressure: Typically around 9-10 atm.
- Rated Burst Pressure: Up to 16 atm.
- SYNERGY™ Everolimus-Eluting Platinum Chromium Stent:
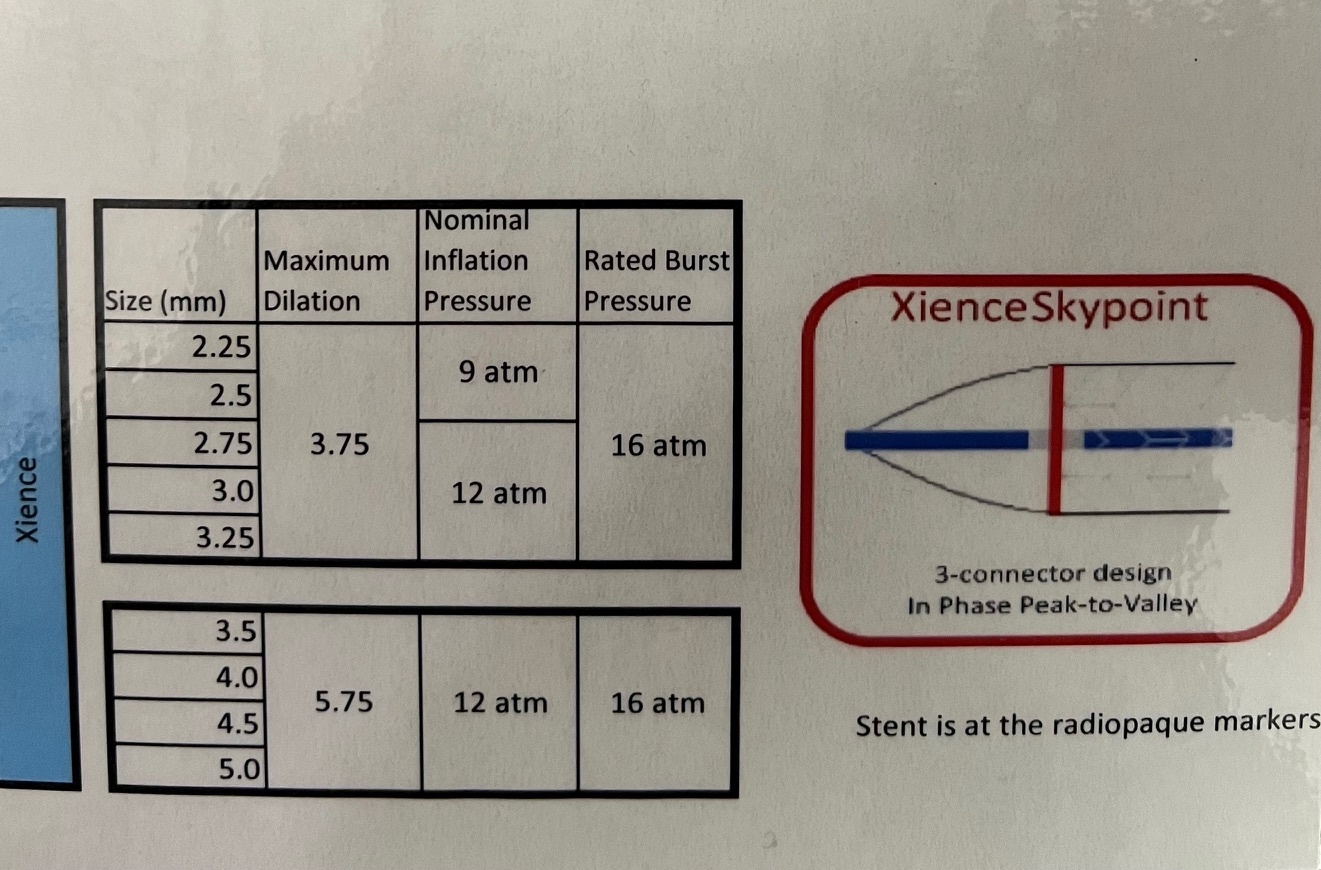
- Abbott Laboratories:
- XIENCE Sierra™ Everolimus-Eluting Coronary Stent System:
- Diameters: 2.25mm to 4.0mm.
- Lengths: 8mm to 38mm.
- Strut thickness: 81µm.
- Durable fluoropolymer coating.
- Nominal Pressure: Approximately 8 atm.
- Rated Burst Pressure: Up to 18 atm.
- XIENCE Sierra™ Everolimus-Eluting Coronary Stent System:
- Medtronic:
- Resolute Onyx™ Zotarolimus-Eluting Coronary Stent System:
- Diameters: 2.0mm to 5.0mm.
- Lengths: 9mm to 38mm.
- Strut thickness: 81µm to 91µm.
- Continuous sinusoid technology for flexibility.
- Nominal Pressure: Around 9 atm.
- Rated Burst Pressure: Up to 16 atm.
- Resolute Onyx™ Zotarolimus-Eluting Coronary Stent System:
Operators should consult the specific stent's compliance chart provided by the manufacturer to understand the expansion capabilities at various inflation pressures. This ensures the stent is appropriately sized to the vessel diameter, minimizing complications like under-expansion or vessel injury.
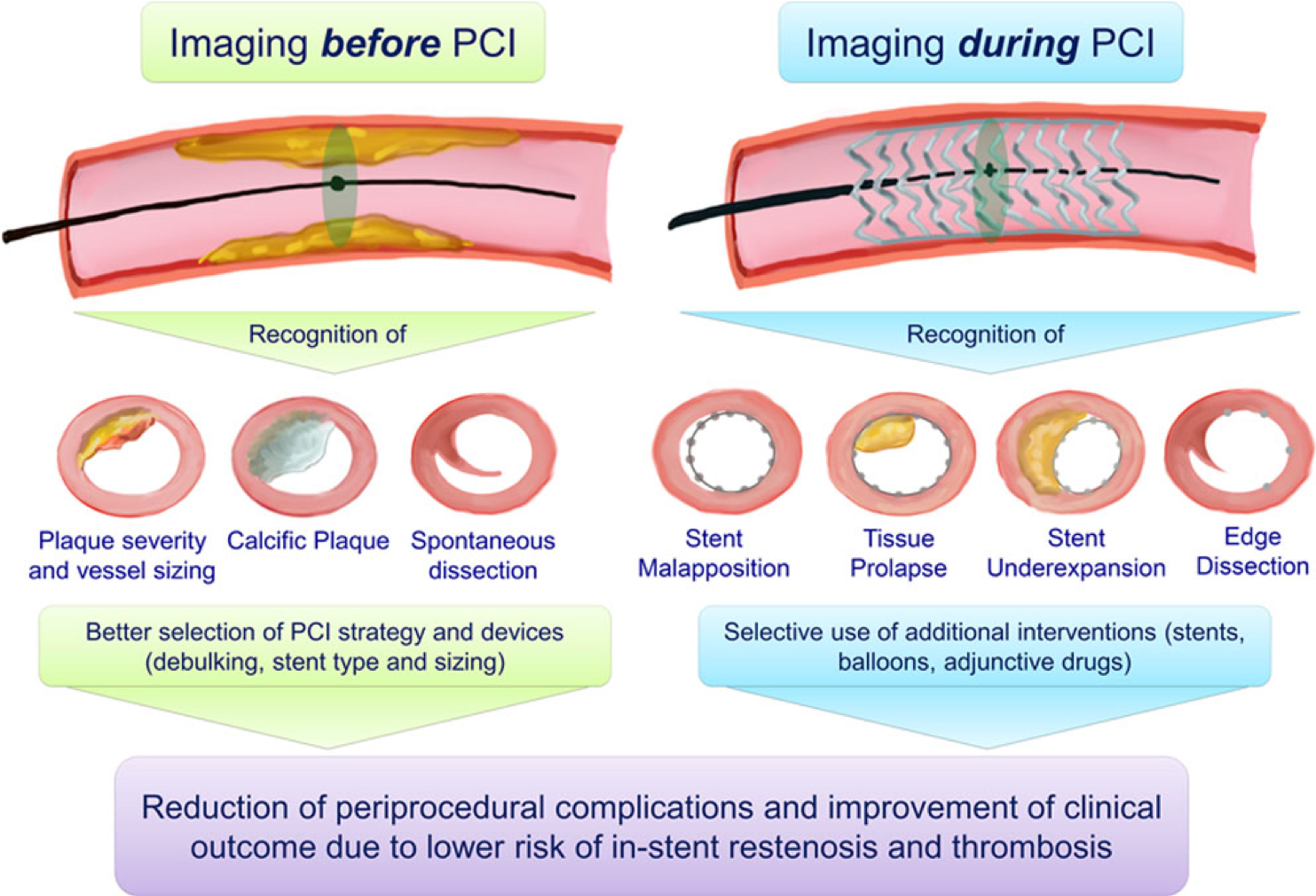
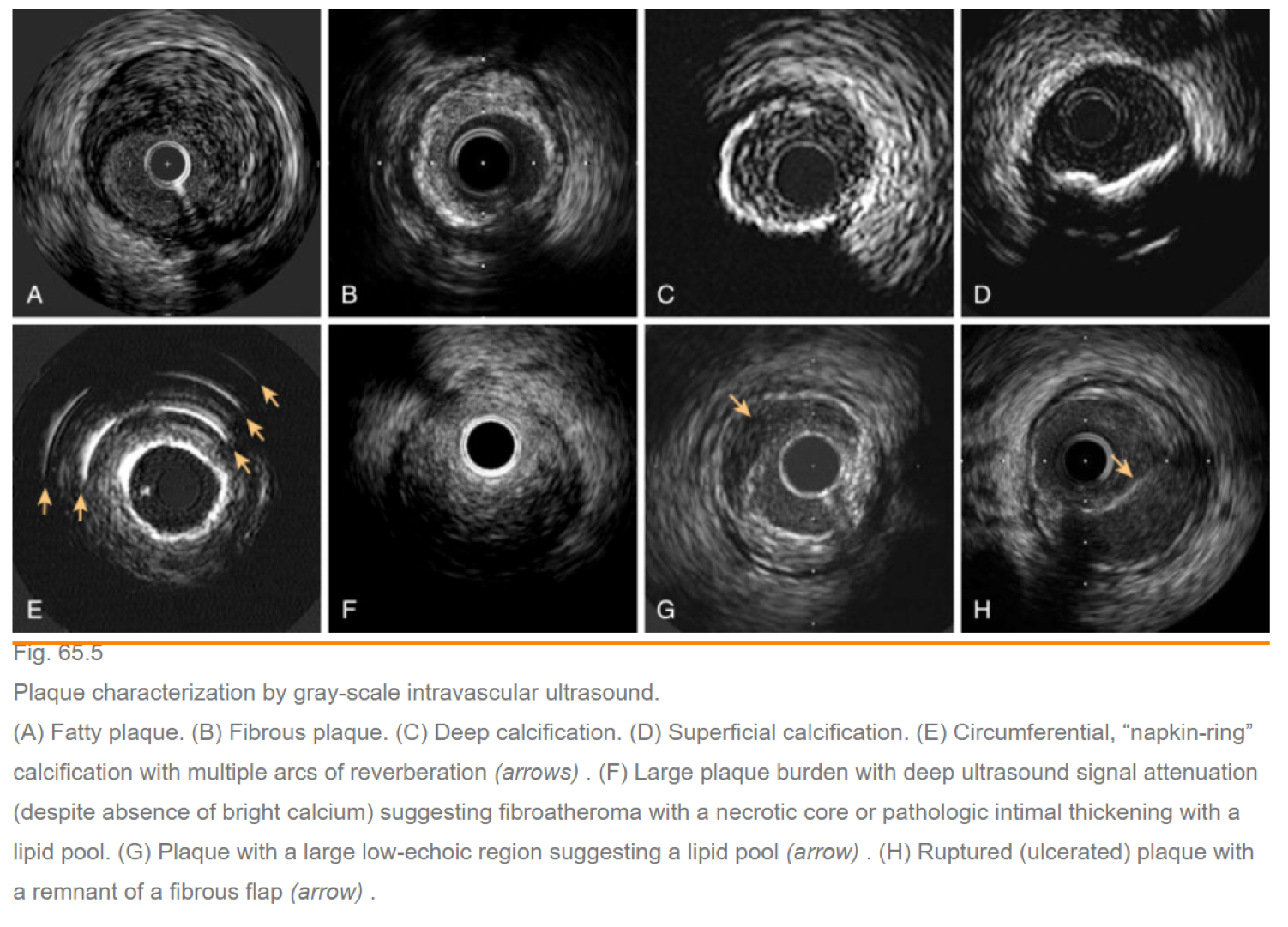
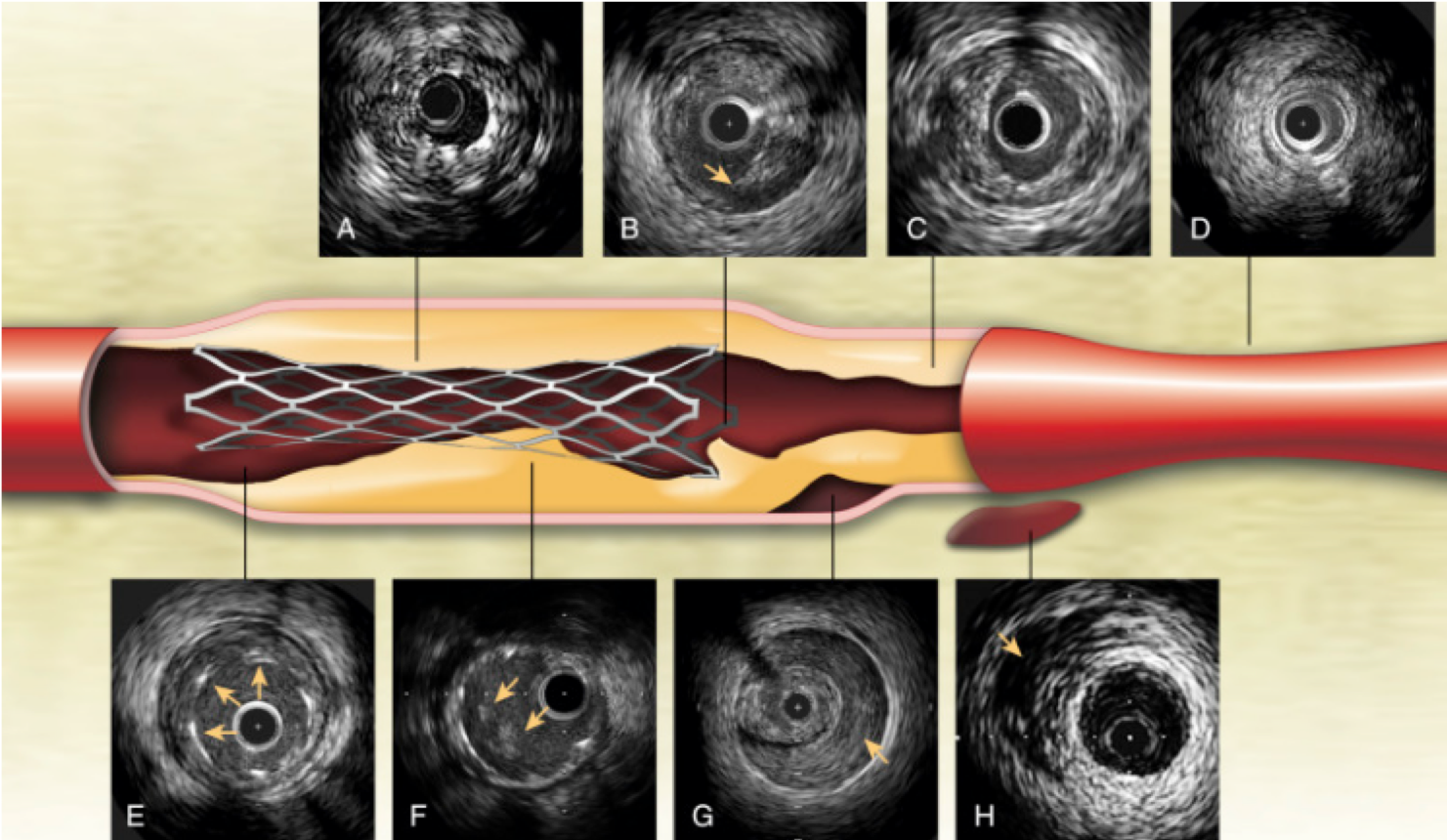
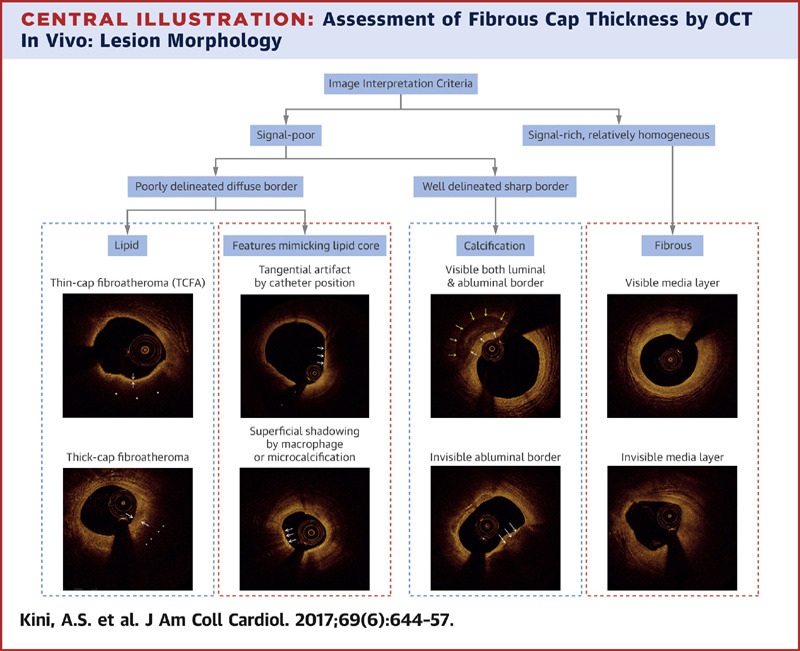
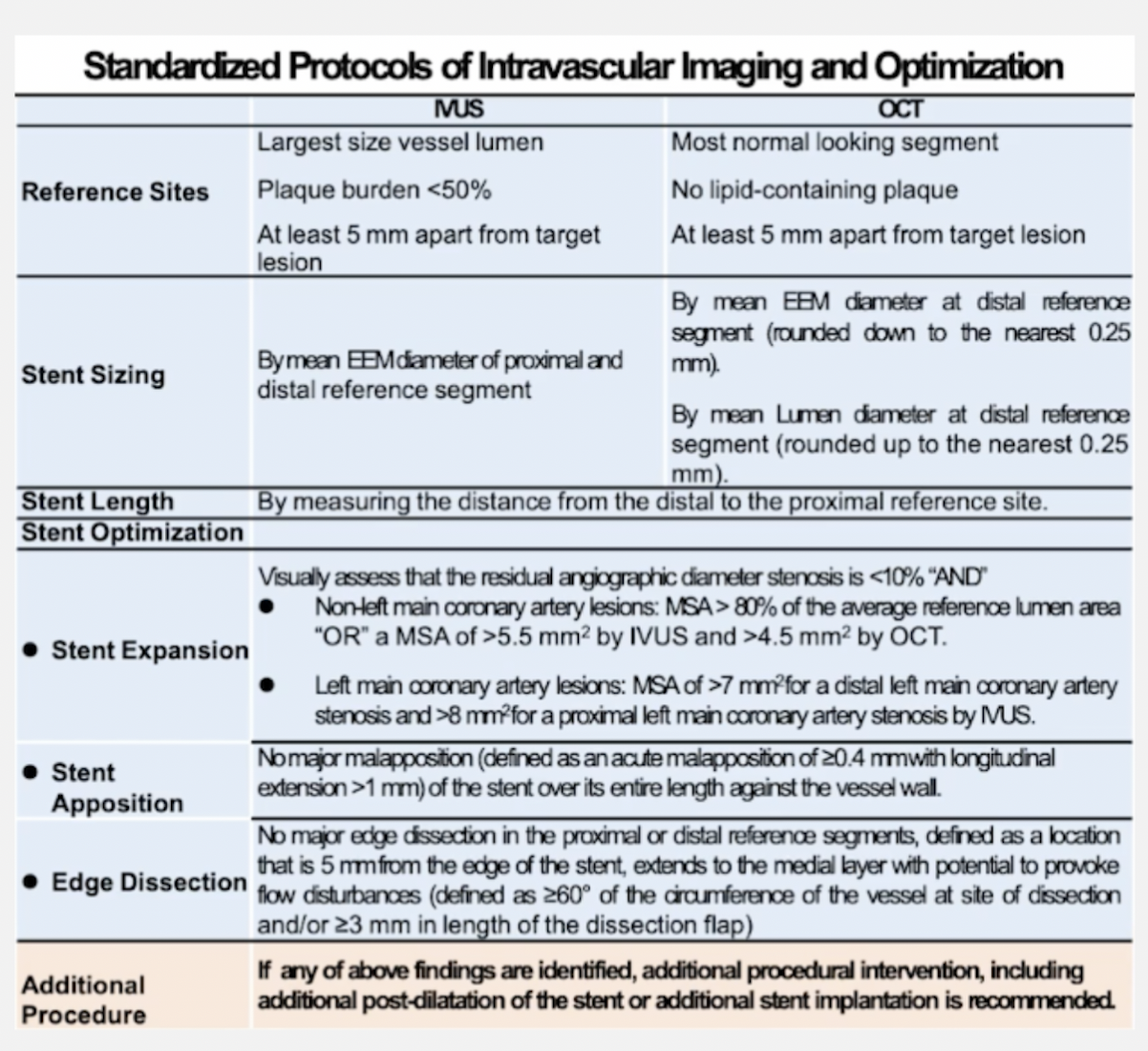
Intravascular Ultrasound (IVUS) and Optical Coherence Tomography (OCT) Intracoronary Imaging
IVUS and OCT are intracoronary imaging modalities used to visualize the coronary arteries from within, providing detailed information about plaque composition, vessel size, and morphology to guide interventional procedures.
Equipment Available
- Intravascular Ultrasound (IVUS) Systems:
- Philips Volcano IVUS:
- Eagle Eye Platinum Catheters (5F compatible)
- Ability to perform virtual histology (VH-IVUS)
- Boston Scientific IVUS:
- OptiCross HD Catheters (compatible with 0.014" guidewire)
- Philips Volcano IVUS:
- Optical Coherence Tomography (OCT) Systems:
- Abbott OCT (Optis Integrated System):
- Dragonfly OPTIS Imaging Catheter
- High-resolution imaging up to 10-20 microns
- Terumo OCT (Lunawave):
- Fast imaging with Primescore OCT Catheter
- Abbott OCT (Optis Integrated System):
- Assess vessel size, plaque characteristics, and lesion length.
- Identify calcifications, thrombus, or dissections.
- Use measurements to guide stent sizing and placement.
- Repeat IVUS or OCT after stent deployment to assess stent apposition and expansion.
- Identify any edge dissections or residual stenosis.
Coronary Hemodynamic Assessment (FFR and iFR)
Coronary hemodynamic assessment using FFR and iFR helps determine the physiological significance of coronary artery lesions, guiding the need for revascularization.
Equipment Available
- Pressure Measurement Systems:
- Philips Volcano:
- ComboMap Pressure System
- PressureWire™ X Guidewire (0.014" compatible)
- Supports both FFR and iFR measurements
- Abbott:
- PressureWire™ X Guidewire (0.014" compatible)
- Optis Integrated System for FFR
- Supports FFR measurements
- Boston Scientific:
- Comet™ II Pressure Guidewire
- Compatible with the OmniWire™ System
- Supports FFR and iFR measurements
- Philips Volcano:
- Hyperemic Agents (for FFR):
- Adenosine (intravenous or intracoronary)
- Regadenoson (if available)
- Equalization of Pressure:
- Zero the pressure transducer to atmospheric pressure.
- Perform pressure equalization:
- Ensure the pressure wire sensor is at the guiding catheter tip (in the aorta).
- Equalize the pressure readings between the wire and the guide (Pd/Pa ratio should be 1.0).
- Pressure Wire Advancement:
- Advance the pressure wire across the target lesion into the distal coronary artery.
- Confirm stable distal position with good pressure signal.
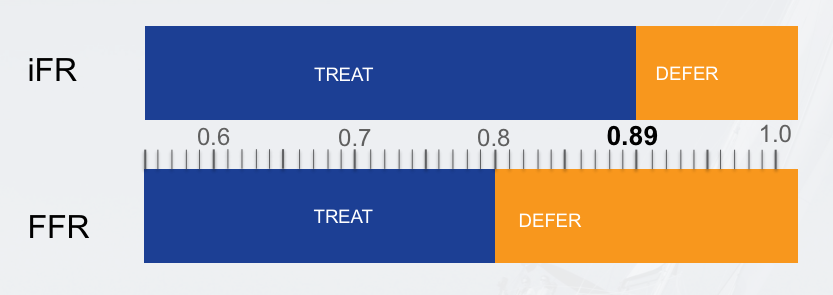
- Baseline Measurement (iFR):
- Measure the instantaneous wave-free ratio during the diastolic wave-free period.
- No hyperemic agent required for iFR.
- An iFR value ≤ 0.89 typically indicates a functionally significant lesion.
- Hyperemic Measurement (FFR):
- Induce maximal hyperemia:
- Intravenous Adenosine:
- Dosage: 140 mcg/kg/min infusion via peripheral or central line.
- Ensure infusion runs for at least 2 minutes before measurement.
- Intracoronary Adenosine:
- Dosage: 100-200 mcg for left coronary artery; 40-80 mcg for right coronary artery.
- Administer as a rapid intracoronary bolus.
- Intravenous Adenosine:
- Record the FFR value during steady-state hyperemia.
- An FFR value ≤ 0.80 typically indicates a functionally significant lesion.
- Induce maximal hyperemia:
- Data Interpretation:
- Compare distal coronary pressure (Pd) to aortic pressure (Pa).
- Assess the need for revascularization based on FFR/iFR values and clinical context.
Atherectomy Devices
Atherectomy devices are employed to modify or remove plaque, particularly in heavily calcified or fibrotic lesions where traditional balloon angioplasty is insufficient.
Rotational Atherectomy
Rotablator™ Rotational Atherectomy System by Boston Scientific:
- Utilizes a diamond-coated burr rotating at speeds of 135,000 to 200,000 rpm.
- Ablates calcified plaque into microparticles (<5 microns).
- Burr sizes range from 1.25mm to 2.50mm.
- Indicated for lesion preparation in calcified coronary arteries.
Orbital Atherectomy
Diamondback 360® Coronary Orbital Atherectomy System by Cardiovascular Systems Inc. (CSI):
- Features an eccentrically mounted diamond-coated crown.
- Orbiting action creates a luminal space while preserving healthy tissue.
- Crown sizes available in 1.25mm and 1.50mm.
- Operates at speeds of 80,000 to 120,000 rpm.
Laser Atherectomy
ELCA® Coronary Laser Atherectomy Catheter by Philips Volcano:
- Emits ultraviolet laser energy to ablate plaque, thrombus, and fibrotic lesions.
- Effective for in-stent restenosis and chronic total occlusions (CTOs).
- Catheter sizes range from 0.9mm to 2.0mm.
Coronary Intravascular Lithotripsy (IVL)
Shockwave™ Coronary IVL System by Shockwave Medical:
- Uses sonic pressure waves to fracture both superficial and deep calcium within the vessel wall.
- Consists of a semi-compliant balloon catheter with integrated lithotripsy emitters.
- Available in diameters from 2.5mm to 4.0mm and lengths of 12mm.
- Delivered like a standard balloon and activated to emit pulses at low pressures (4 atm).
IVL is particularly beneficial for lesions with heavy circumferential calcification, facilitating optimal stent expansion and apposition.